Autoimmunity and tolerance
by Jessica Rose | Jul 10, 2022
During my Immunology degree program, we used many textbooks to enhance our understanding of all things immunity. Kuby, Janeway, Parham, Wood – each one with a bunch of pros and cons. I liked Kuby. Janeway was always the go-to. The latter written by Peter Wood called “Understanding immunology”1 has a chapter in it called “Autoimmune diseases”. In this chapter, autoimmunity is defined and classified with regard to T cell and antibody responses, the aetiology is explored with regard to genetic and environmental factors and perhaps most importantly: loss of tolerance is discussed.
On autoimmune diseases
Autoimmune diseases are diseases that involve an immune response against one or more self-antigens or auto-antigens.
Auto-antigens are molecules that comprise you. So it’s an immune attack on you. Why would your body turn on itself?
I screenshotted (is that a word?) page 210 of the textbook that has a list of autoimmune diseases recognized at the time this book was written and published.

Figure 1: Understanding immunology. Table 12.1. Page 210.
The list comprises autoimmune diseases involving the kidney, joints, skin, nervous system, haematopoietic system and endocrine glands. Most people know about Rhematoid arthritis: this is a classic autoimmune condition involving progressive destruction of the synovium (the soft tissue that lines human joints). One of the diseases listed here is called Pemphigus vulgaris. This involves an autoimmune reaction against the epidermal cell junctions to induce severe blistering. You guys might be interested to know that in the context of the COVID-19 injectable products in VAERS, there are currently (as of July 8, 2022) 97 reports of Pemphigus vulgaris.
Figure 2: Pemphigus vulgaris photographs. https://search.brave.com/images?q=Pemphigus%20vulgaris
Pretty gross, huh.
Speaking of VAERS, the list of autoimmune diseases reported in the context of these injections is very long. Much longer than the list in this textbook.
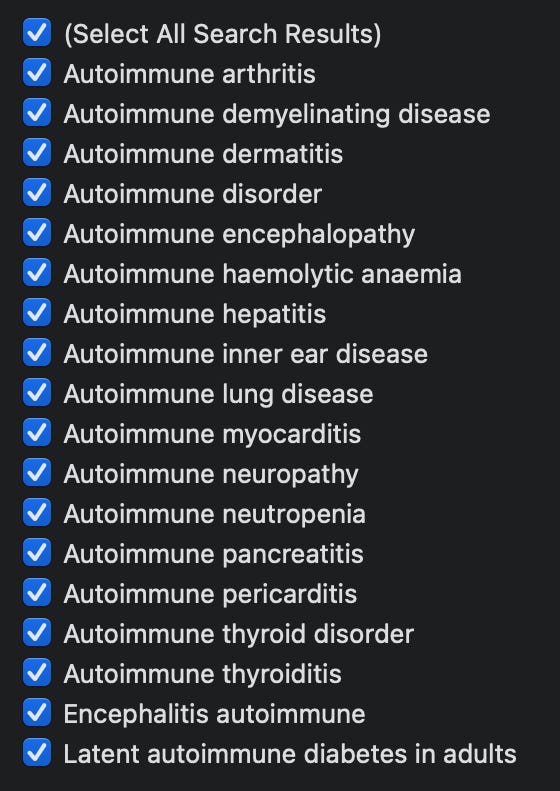
Figure 3: A sample of some of the reported adverse events in VAERS by MedDRA code
On tolerance
There are two kinds of immunological tolerance: central and peripheral. Central tolerance occurs in the places where T and B cells are generated: the (T)hymus and (B)one marrow. These are called primary lymphoid organs because these cells essential to adaptive immunity come from these organs. The antigens that are ‘handled’ are those expressed in cells in the thymus or on cells trafficking through the thymus. Peripheral tolerance occurs in the periphery and comprises cells circulating in the periphery and peripheral organs like the liver and heart.
The tolerance part is the removal of T or B cells that are capable of recognizing self-antigens. This occurs by a number of different mechanisms.
On central tolerance mechanisms
- Clonal deletion involves physical removal of a cell.
- Clonal inactivation involves the peripheral inability to mount a response.
- Clonal diversion involves identity removal of effector properties to become a different kind of cell like a regulatory cell.
On peripheral tolerance mechanisms
- Clonal deletion involves physical removal of a T cell.
- Clonal inactivation involves the peripheral inability to mount a response.
- Clonal diversion involves identity removal of effector T properties to become a different kind of cell, like a regulatory T cell.
- Clonal ignorance involves antigens that reside in immune privileged sites like the eyes, brain and testes and thus cannot be seen by circulating lymphocytes.
- Clonal helplessness involves the inability to get help from helper T cells which have been tolerized.
- Clonal suppression involves cells that keep effector T cells in check.
Clonal deletion involves removal of self-reactive T cells from the T cell repertoire via apoptosis. About 2/3 of all thymic mature T cells will be deleted due to self-reactive properties! The way this deletion occurs is primarily by anergy or apoptosis. Anergy is when a cell becomes useless and cannot ‘effect’, and apoptosis is when the cell self-destructs.
Depending on which type of MHC molecule the T cell has, and depending on which a peptide is mounted on it, that cell will become a helper T cell or a cytotoxic T cell depending on this antigen presentation context. This is important to tolerance since these very same receptors are found on pre-cursor versions of these T cells as they get educated in the thymus. This involves rearrangement of the T cell receptor genes and phenotypic specialization – the decision to be either an effector cell or a regulatory cell. This occurs by two types of selection: positive and negative selection whereby cells are selected to live on, or selected to be deleted. The enrichment of T cells that occurs in the thymus that are able to see the MHC molecule that is expressed, but not too well, to create the pool of cells will ultimately be in circulation and not auto-reactive!
Pre-cursors from the fetal liver or adult bone marrow enter the thymus and percolate through the thymic cortex where they contact many different stromal cell types.
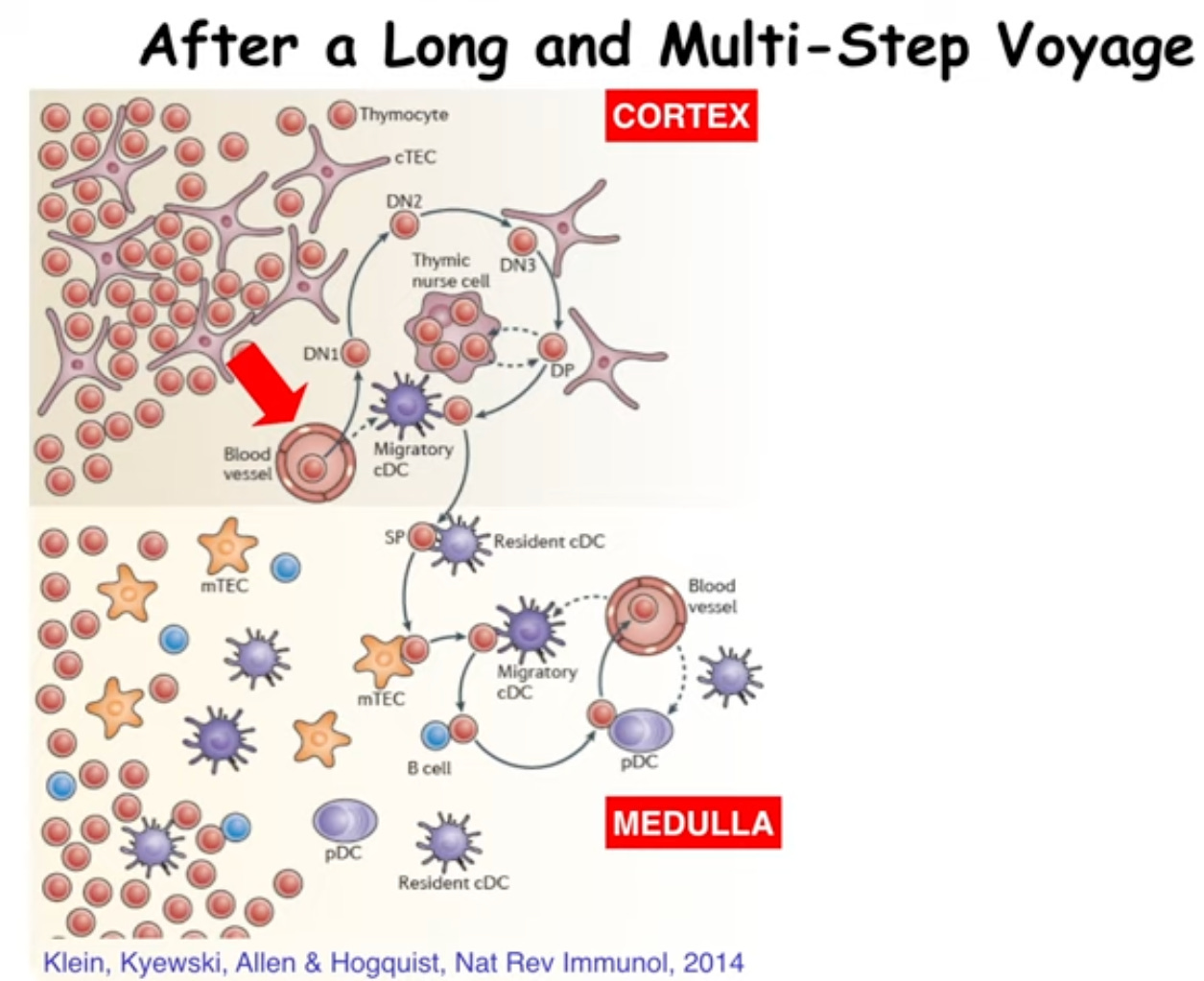
Figure 3: iBiology lecture. An Introduction to T Cell Tolerance.
These special interactions induce the outcome of the T cell: will it stay or will it go? The final maturation of these cells via selection determines their fate as well: will I be a CD4 or a CD8? This depends on affinity and avidity of interactions with other cells. It’s just like Goldilocks: the amount and the strength of interaction must be ‘just right’ or the cell will be removed.
So we all end up with T and B cell repertoires that allow us potent specific responses against antigens but without responses aimed at self. Unless… a break in tolerance occurs.
An assault on tolerance?
When breaks in tolerance occur, this leads to autoimmunity. So although it seems as though this should rarely occur due to all of these amazing tolerance mechanisms, rare has more recently become, not so rare.
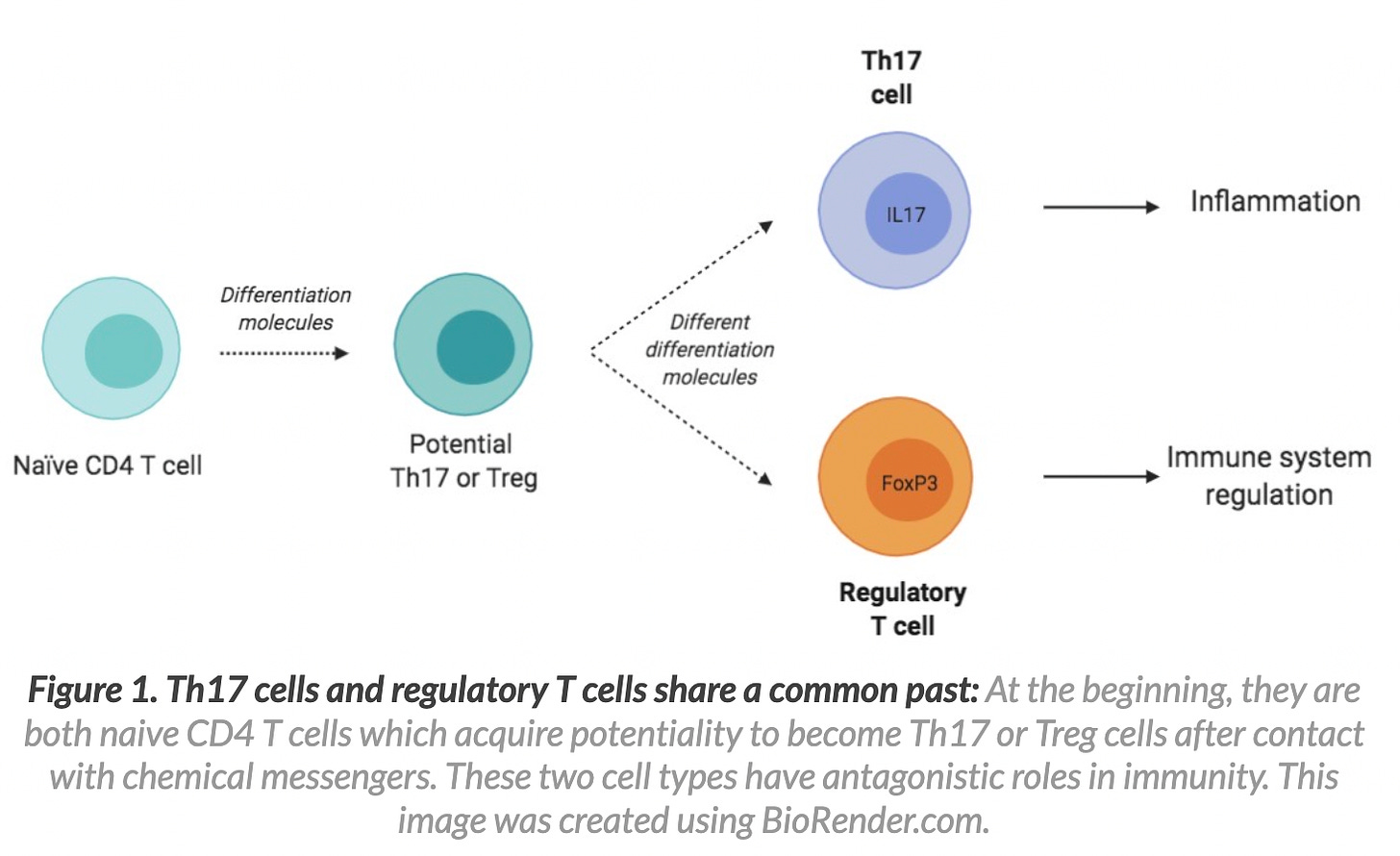
So what causes a break? Well, to answer we need to learn about a population of T cells known as regulatory T cells. Regulatory T cells are a special subpopulation of T cells that regulate immune responses to keep other cells in check (they can down-regulate induction and proliferation of effector T cells for example and used to be called suppressor cells). They are vital to maintain tolerance to self-antigens. Loss of a particular population of regulatory T cells (Tregs for short) induces severe autoimmunity. FoxP3+CD4+ are a dedicated and famous population of regulatory T cells. They circulate throughout the body at around 10% of the total circulating T cell population. In the absence of this special population of T cells, you get autoimmune diseases.2 3 4 5 6 7
I wonder. Could it be that the spike protein does something to FoxP3+CD4+ Tregs? Maybe the high IL-6 induction in the context of SARS-nCoV-2 and COVID-19 (and potentially from the spike protein alone) causes a skew in Th17 cells and a reduction in Tregs to induce autoimmunty?8 9 10
A study (reference 8) of the potential differences in the functionality of Th17 and Treg cells in SARS-CoV-2 patients compared with healthy controls showed the following:
The findings revealed a significant increase in the number of Th17 cells, the expression levels of related factors (RAR-related orphan receptor gamma [RORγt], IL-17, and IL-23), and the secretion levels of IL-17 and IL-23 cytokines in COVID-19 patients compared with controls. In contrast, patients had a remarkable reduction in the frequency of Treg cells, the expression levels of correlated factors (Forkhead box protein P3 [FoxP3], transforming growth factor-β [TGF-β], and IL-10), and cytokine secretion levels (TGF-β and IL-10).
So SARS-CoV-2 patients are associated with fewer T regulatory cells. So it is safe to say that these people might be expected to experience re-establishment or existing autoimmune diseases or the development of new ones. This finding is repeated in the literature.
SARS-CoV-2 invasion induces significantly increased level of serum IL-6, which promotes the differentiation of naïve CD4+ T cells into Th17 cells as well as inhibits the expression of Tregs, resulting in an imbalance of the Treg/Th17 ratio.11 12 In addition, some COVID-19 patients develop other complications such as diabetes, which cause CD4+ T cells to differentiate into Th1 and Th17 rather than into Tregs, leading to diminished immunosuppression.13 (From reference 5.)
So loss of tolerance can happen with Tregs are not around and doing what they are meant to do which is to control autoimmune diseases and tumor escape, for example. This happens in the presence of too much IL-6, which unfortunately, is the status quo for people with COVID-19. So is this the same deal with the spike shots?
It is frightening to me, once again, how very off kilter these injections seem to throw so many components of our beautiful immune systems in so many people.
I think this Substack article took on a life if its own. There’s so much to cover here but I will close it up with a research question:
Question: Is the spike protein manufactured upon injection with COVID-19 products inducing autoimmunity via upregulation of IL-614 and subsequent induction of differentiation of naïve CD4+ T cells into Th17 to perturb the Treg/Th17 ratio to re-establish or cause autoimmune diseases and tumor progression?
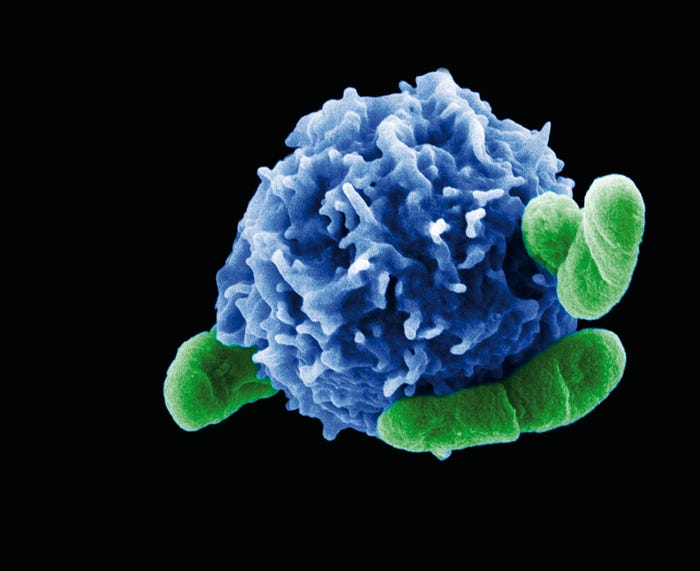
Figure 6: A regulatory T cell interacting with bacteria. https://www.labroots.com/trending/immunology/2324/protective-autophagy-in-regulatory-t-cells
To be continued…
Subscribe to Unacceptable Jessica
Peter Wood. Understanding immunology. Cell and molecular biology in action series. Prentice Hall. Published by Pearson Education. 2001. ISBN 0-582-32731-8.
Uraki R, Imai M, Ito M, Shime H, Odanaka M, Okuda M, Kawaoka Y, Yamazaki S. Foxp3+ CD4+ regulatory T cells control dendritic cells in inducing antigen-specific immunity to emerging SARS-CoV-2 antigens. PLoS Pathog. 2021 Dec 9;17(12):e1010085. doi: 10.1371/journal.ppat.1010085. PMID: 34882757; PMCID: PMC8659413.
Meckiff BJ, Ramírez-Suástegui C, Fajardo V, et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell. 2020;183(5):1340-1353.e16. doi:10.1016/j.cell.2020.10.001.
Gao M, Liu Y, Guo M, et al. Regulatory CD4+ and CD8+ T cells are negatively correlated with CD4+ /CD8+ T cell ratios in patients acutely infected with SARS-CoV-2. J Leukoc Biol. 2021;109(1):91-97. doi:10.1002/JLB.5COVA0720-421RR.
Wang H, Wang Z, Cao W, Wu Q, Yuan Y, Zhang X. Regulatory T cells in COVID-19. Aging Dis. 2021;12(7):1545-1553. Published 2021 Oct 1. doi:10.14336/AD.2021.0709.
Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239-244. doi:10.1038/ni1572.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490-500. doi:10.1038/nri2785.
Sadeghi A, Tahmasebi S, Mahmood A, et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J Cell Physiol. 2021;236(4):2829-2839. doi:10.1002/jcp.30047.
Tahmasebi S, Saeed BQ, Temirgalieva E, et al. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. 2021;276:119437. doi:10.1016/j.lfs.2021.119437.
Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2(Suppl 2):S3. doi: 10.1186/ar1917. Epub 2006 Jul 28. PMID: 16899107; PMCID: PMC3226076.
Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH (2020). COVID-19 and Treg/Th17 imbalance: Potential relationship to pregnancy outcomes. Am J Reprod Immunol, 84(5):e13304.
Lee GR (2018). The Balance of Th17 versus Treg Cells in Autoimmunity. Int J Mol Sci, 19(3):730.
Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H (2013). The plasticity and stability of regulatory T cell. Nat Rev Immunol, 13(6):461-7.
Interleukin (IL)-6 is produced at the site of inflammation and plays a key role in the acute phase response as defined by a variety of clinical and biological features such as the production of acute phase proteins. IL-6 in combination with its soluble receptor sIL-6Rα, dictates the transition from acute to chonic inflammation by changing the nature of leucocyte infiltrate (from polymorphonuclear neutrophils to monocyte/macrophages). In addition, IL-6 exerts stimulatory effects on T- and B-cells, thus favoring chronic inflammatory responses. Strategies targeting IL-6 and IL-6 signaling led to effective prevention and treatment of models of rheumatoid arthritis and other chronic inflammatory diseases. From reference 10.


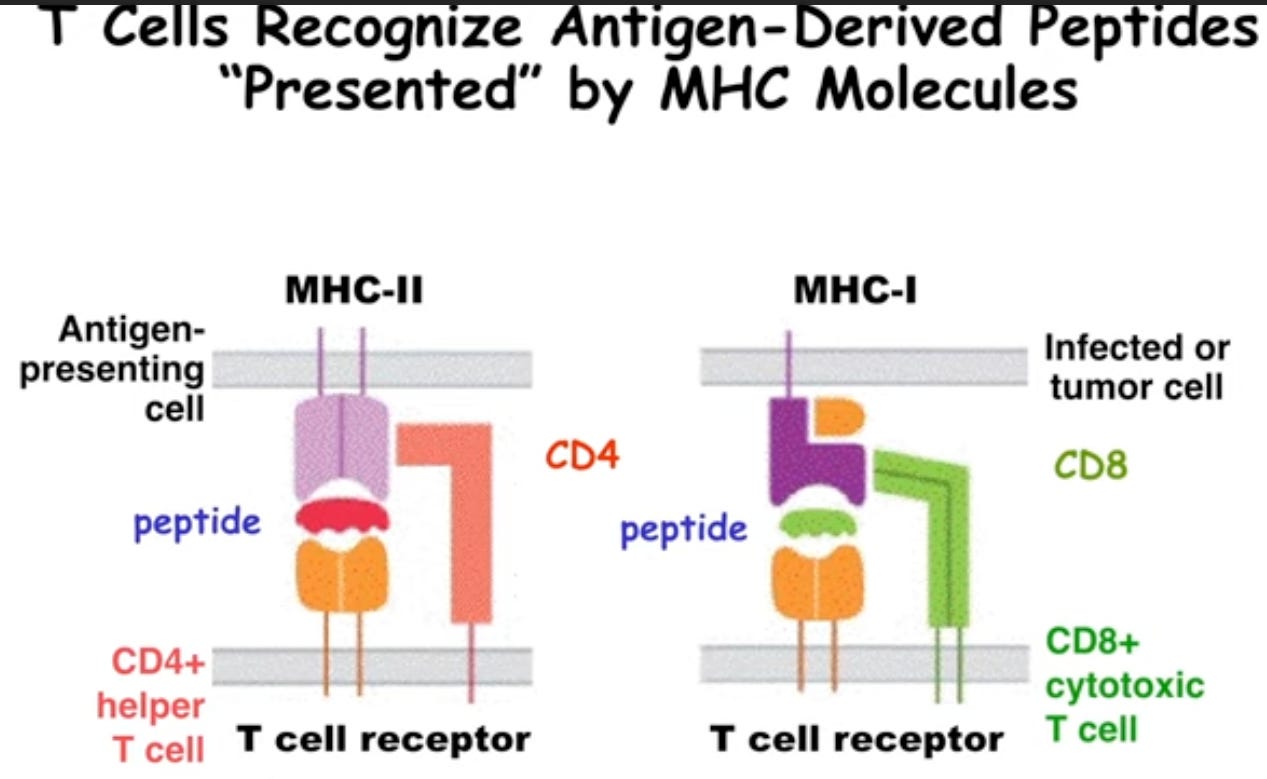 Figure 2: iBiology lecture. An Introduction to T Cell Tolerance.
Figure 2: iBiology lecture. An Introduction to T Cell Tolerance.



0 Comments