Subscribe to Zero-Sum Pfear & Loathing


In the first part of this series, I discussed the forgotten side of water and the immense complexity that molecules of water can demonstrate as a group. Sadly, while ample evidence exists that there is more to water than we realize, the scientific community has steadfastly refused to acknowledge this phenomenon, and how this widespread denial mirrors many of the problems we have faced throughout COVID-19. I would highly advise reading it first as it lays the context for the biological consequences of EZ (liquid crystalline) water that will be discussed here.
Note: as discussed in the previous article, since many people have observed the liquid crystalline state of water, a variety of different terms are used to discuss it.
In that article, I reviewed the lineage of scientists who, for over a century, all came to a similar conclusion: water can exist in a structured state where it behaves like a liquid crystal. Most of them figured this out by observing cells and noticing water within cells, especially that the water in the vicinity of a cellular surface (e.g., a protein) was different from the normal (termed “bulk”) water we are used to working with.
Gerald Pollack built upon this century of observations, and eventually determined that when a negatively charged surface is present, and ambient electromagnetic energy such as light exists (to some extent sound can also fulfill this role), the water will assemble itself into layers of offset hexagonal sheets with the formula H3O2. This structure has a significant degree of solidity, and will expel most things from entering it (e.g., polystyrene microspheres), including the displaced hydrogen atoms (as it is H1.5O not H2O). These displaced positively charged H atoms (henceforth referred to as protons), in turn assemble immediately outside this lattice, thereby creating a pH and charge gradient which can be measured.
In many cases, this H3O2 structure can be enormous—in favorable conditions, Pollack and others have measured ones ranging from 0.1 millimeters to 0.5 millimeters (100,000 to 500,000 nanometers or nm) in size. Cells depend on this water, so they contain a large number of surfaces from which the water can form. For example, 20% of the cell is occupied by its cytoskeleton (a protein lattice which maintains its structure), and analysis of high-voltage electron micrographs have shown that within the cytoskeleton, over half of the water present is within 5 nm of a surface it could potentially form H3O2 on (note: a H2O molecule is 0.27 nm in size).
Since the structuring surface sites for (H2O) water are so closely packed together in cells, it is understandable why liquid crystalline (EZ, H3O2) water would be so much more apparent to individuals observing cells than workers performing non-cellular work observing water under a microscope. This in turn raises a different question: why are cells designed to create so much liquid crystalline water (H3O2)?
In the previous article, I discussed a common issue I observe within science. When an incorrect model is utilized to explain a natural process, discrepancies between the model and reality will inevitably appear. One would expect that when this happens, it would encourage those espousing the erroneous model to re-examine their model, but instead, since so much has been invested into that model, they instead will denounce any challenges to it, and come up with innumerable creative ways to explain away each failure of the existing model.
Consider, for example, the initial promises of the vaccines (if you get two doses, you are told that you will be completely immune, transmission will stop, and COVID-19 will rapidly fade into memory). Since the clinical trials for the vaccines were fraudulent, none of the vaccines’ promises materialized, and the COVID situation instead, became worse. However, instead of healthcare authorities (and the medical community) admitting their mistake and switching to a different approach for managing COVID-19, they doubled down on the vaccine mandates and moved the goal posts more times than I can count on exactly what mass-forced vaccination (and boosting) was supposed to accomplish.
Likewise, with cell biology, our knowledge of the cell is surprisingly primitive and the existing models often fail to explain what occurs within the cellular environment. However, since better models are not within the grasp of the scientific community, we have been forced to continually patch the existing models so that they can account for the innumerable mysteries of life.
At this point, I believe one of the key causes of this situation is scientific research becoming distorted to prioritize focusing on discoveries that industry can profit from. For example, immunology holds a narrow focus on the aspects of the immune system, which can be targeted by vaccination or proprietary drugs, and because this has left many other components of the immune response neglected, leading to it being commonly referred to as one of the least understood systems in the body. Similarly, since pharmaceutical drugs often work by affecting receptors and channels in cells, cellular biology has adopted a narrow-minded focus on those aspects of a cell.
Fortunately, the liquid crystalline (EZ) phase of water provides a variety of explanations for many of the phenomena that the existing models do not adequately address. In this article, I will focus upon a few of them.
Note: Much of what is discussed in this article is covered in more detail within these three books (similarly, the majority of the references for this article are sourced from these books, so I will not repetitively cite them throughout the article). If you wish to further study the subject yourself, I would recommend reading those books (all authored by Gerald H. Pollack) in this order:
•The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor (2013)
•Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function (2001)
•Phase Transitions in Cell Biology (2008)—this is the most technical of the three.
One of the major puzzles of biology is the immense durability that cells have. If you consider the classic model—cells being bags of liquid contained within a fluid mosaic membrane, it should be effortless for external forces to “pop” cells and have all of their contents spill out. Yet in most cases, cells maintain their integrity despite significant stressors.
Cells can survive traumas, including being guillotined in half, drawn and quartered (so specific components can be isolated and worked with—such as when performing in vitro fertilization), or shot full of holes with electrical bullets, each of which one would expect would be sufficient to “pop” them. However, in each instance, cellular integrity of the remaining component persists.
Similarly, if the membrane from a cell is removed, its internal contents remain in place rather than rapidly disappearing. It has also been known for over 50 years that if muscle fibers lose their membranes, their functional ability (creating a contractile force) remains intact.
Three clues help to explain these phenomena. The first is that—as Pollack has shown—water droplets have a remarkable amount of integrity, and like cells, will maintain their integrity (and subsequently fuse back together) if a micro-blade is used to guillotine them in half. The second is that Pollack has also shown water droplets contain a significant degree of liquid crystalline water which likely is what confers their integrity. The third is that the water molecules in cells predominantly exist within gels, which are composed of that same liquid crystalline water. Put differently, this means that a cell’s stability is largely a property of its water holding it together rather than the external structure which encapsulates it.
Another important aspect of cellular architecture should now be considered. As the conditions for liquid crystalline water formation are present throughout the cell (negatively charged hydrophilic surfaces and ambient infrared energy), the cells should rapidly be filling themselves with liquid crystalline water layers hundreds of micrometers in thickness. Yet, throughout the cells, the surfaces are often only fractions of a micrometer apart. This means that the structure of the cell depends upon the growth of liquid crystalline water, but simultaneously constrains that crystalline structure from growing to its full size.

This model often does not work within living organisms, because life, unlike those buildings, requires rapid movement, and the organisms simply cannot produce solid structures with the same strength as steel beams. However, an alternative and more complex architectural model has been developed which is frequently utilized by those who embrace complexity.
Tensegrity (short for tensional integrity), was a model first put forward by Buckminster Fuller. It posits that if a series of non-compressible structures are linked together by a lattice of elastic connections (which can store tension when stretched), a much stronger structure is created. This is because any force the structure receives will be equally distributed through each of those elastic connections rather than concentrating on a single component (e.g., the stone pillar), and thus, much less likely to exceed the breaking point of any single structural component.
Fuller’s work subsequently inspired numerous buildings to be built on the principles of tensegrity. This is a classic picture of him holding a tensegrity sphere he made:
Biotensegrity encapsulates the realization that this same system also occurs throughout nature. For the human body, at each level of organization, a linked tensile matrix is present that confers its stability. One French hand surgeon, Jean-Claude Guimberteau, has likewise done a remarkable job of demonstrating the presence of linked tensile structures at the level of fascia (a connective tissue present throughout the body many manual therapists work with), through small (magnified) cameras placed in the body during minimally invasive surgeries:
Note: at the magnification scale used here and in his other videos, liquid crystalline water can be directly observed coating these structures. Also note its lubricating quality that allows the structures to slide past each other. This lubrication is extremely important and a variety of problems set in when it is lost.
As the years go by, there appears to be a greater consensus within the holistic medical field that biotensegrity is a valid model for understanding the body, and that linked networks of tension are present from the largest to the smallest levels of the body (e.g., the cytoskeleton is the elastic connecting unit within each cell). However, while this theory is frequently discussed, there are still two major unaddressed issues with it, which I believe liquid crystalline water can explain.
The first is that in Guimberteau’s work, he had observed that tiny vacuoles (enclosed compartments filled with water) throughout the body form the basic incompressible unit that much of the body’s tensegrity depends upon. The second is that at an even higher magnification within cells (which also lack a clearly defined incompressible unit), while an elastic cytoskeleton is present, nothing is visibly holding it under tension (it is highly debatable if the cell’s connections to the extracellular matrix suffice to do this). This matters because the structural strength of tensegrity only emerges when a structure’s components are under tension.
In the case of the former, I believe that the microvacuoles filled with liquid crystalline (EZ) water (thus creating their incompressibility). In the case of water droplets, Pollack concluded that the mutual repulsion between positively charged protons (pushed to the center of the droplet by exclusion zones) creates an outwards force resisted by the liquid crystalline boundary of the droplet. The balance between these two forces results in the droplet assuming a spherical form, and I believe the same mechanism is at work in the microvacuoles throughout the human body.
In the case of the latter, it is important to remember how much expansion is produced by proteins that create gels (many gels are over 99.9% water). Pollack, in turn, has shown that the liquid crystalline water that is constantly trying to form within the cell is unable to reach its full size due to proteins resisting the stretch that would have to occur for the liquid crystalline gel (that the surrounding proteins generate) to be able to reach its full size. This happens both at the level of the cell (as the cytoskeleton stretches as the cell expands to its maximum size, until it cannot allow further expansion to occur) and within proteins throughout the cell.
At the protein level, the body often relies on chemical bonds to be made between proteins to constrain the maximum expansion that can occur in response to a gel forming. Additionally, proteins can alternate between a folded and an unfolded conformation (e.g., a helix vs. a coil), something often determined by the tension applied to the protein (such as the expansive pressure of liquid crystalline water within a gel). There are a variety of importance consequences of this conformation change which will be discussed further with the physiology of muscles.
Additionally, many other components of the body also appear to rely upon liquid crystalline water:
Studying collagen, Melacini et al. noted the importance of water in stabilizing its triple helix crystalline structure. They found that water within the collagen helix forms a “semi-clathrate-like structure that surrounds and interconnects triple helices in the crystal lattice.” Water within the matrix of bone is likewise highly structured, a structuring that has been found associated not only with organic macromolecules such as collagen and proteoglycans, but with the mineral surfaces as well. Water, in fact, seems to play a foundational role in orienting mineral nanoparticles into parallel arrangements within the bone matrix, providing this orientation even in the absence of organic molecules.
Many technologies we are familiar with (e.g., diapers) rely upon hydrogels which can expand into a semisolid structure which retains water. Similarly, we can directly observe that many larger processes within the body are also dependent upon water’s tendency to assemble into the larger liquid crystalline structures.
Because the normal architecture of protein’s cross-linking behavior limits how much gels can expand (as the proteins which would need to separate to accommodate the growing gel are prevented from doing so by the cross links), when tissue or protein is damaged, this limitation can be partially removed. As a result, experiments on a microscopic scale have been conducted showing that human tissue will swell and expand when it is damaged. Likewise, Pollack has argued that this is most likely what happens when you experience a musculoskeletal trauma. In Pollack’s proposed model, the initial gel formation further expands the existing tear, and this progressive expansion of liquid crystalline water eventually leads to visible swelling.
On a larger scale, one of the major engineering challenges for the body is having weight-bearing joints like the knees be able to maintain their range of motion without becoming damaged by the continual friction they experience on a daily basis. A remarkable characteristic about liquid crystalline water is that provided the surface from which it forms remains, it can be destroyed and then reform its almost frictionless surface over and over again.
Because of this, the liquid crystalline water ends up being the component which absorbs the stress experienced by healthy joints, and provided the joint is healthy, this water can instantly regenerate from that stress. In conjunction with this regenerating layer of negatively charged liquid crystalline water, at the very center of the joint, there is a pocket of positively charged protons which repel from each other and create an expansive pressure (like what is seen in a water droplet). Since the joint capsule seals this region, those protons are unable to escape and thus effectively function like repelling magnets (e.g., consider a maglev train) that resist the weight of the body and maintain the central space within the joint.
One of the things I consider most compelling about Pollack’s model for the joints is the specific quality of synovial fluid inside the knee joint. When you view it on a camera during an arthroscopic surgery, diffusion within it is visibly slowed, while if you directly extract it (e.g., during a knee aspiration), you can tell that it has a much thicker and gelatinous quality, another quality I have learned to associate with the presence of liquid crystalline water. Pollack likewise argues that his model suggests EZ (liquid crystalline) water behavior resembles that of a gelatinous egg white which is semisolid when left alone, yet able to flow in response to an imposed shear force.
Note: a “gradient” describes a difference in the concentration of things in two different areas. This could include electrical charges (batteries depend upon gradients), electrolytes, or temperatures.
The existing paradigm of physics supports the following:
•The natural state of things is to be disordered and evenly mixed.
•Anytime you make something become more ordered (e.g., forming a crystal or creating a gradient between two areas), energy must be expended to create that ordering.
•When an ordered structure becomes disordered, energy is released in the process which can sometimes be harvested (e.g., combusting wood to in a wood stove to heat our homes).
•Anytime energy is released from something, if you attempt to capture and store that energy, some energy will always be lost.
These laws, in turn, are used to refute the possibility that any type of “free-energy” type system can exist, such as the exclusion zone (EZ) water system Pollack has proposed. Unfortunately for the existing paradigm, biology often appears to violate these laws, as it is continually moving towards a more ordered state rather than the disordered state the paradigm predicts.
The current resolution for this paradox (which won a Nobel prize) is that living organisms function as “dissipative structures”, which exchange the order in large amounts of ordered components they accumulate from their environment in return for imparting order to their own disordered contents. Although to some extent this allows the existing paradigm to sustain itself, I do not believe it is entirely accurate as water, through its liquid crystalline structure, has the ability to store radiant energy that passes through it and convert that energy to order the body can utilize.
One of the things living cells are well known for doing is concentrating potassium inside themselves and reciprocally expelling sodium. Since the concentration inside and outside cells differs, a gradient exists, which by the existing laws of physics should try to equalize itself and rapidly disappear.
Since this does not happen, the existing model has argued that the cellular membrane prevents the passage of most but not all sodium and potassium (thereby inhibiting the gradient from equalizing itself) and that sodium potassium pumps on the cellular membrane exist which continual swap sodium inside the cell for potassium outside the cell. Because this exchange is so vital to maintaining the health of the cell, a large focus in cellular biology is placed on the importance of the sodium potassium exchange pump.
Unfortunately, there are three fundamental problems with this model (the evidence of each of these and more is presented by Gilbert Ling here):
•The math does not add up—the existing sodium potassium pumps simply do not have the capacity to counteract the natural undoing of the sodium and potassium gradients. For example, in muscle cells, maintaining the sodium potassium gradient with pumps requires between 4 to 30 times the total available energy in the cell.
•Cells are able to maintain a gradient with a variety of other undesirable components they expel (e.g., bacteria expelling antibiotics) and to explain these phenomena, more and more pumps are identified to try to support the model. This is a problem because it is unlikely that cells have the capacity to simultaneously sustain so many different pumps.
•Ling took frog muscle cells whose environment was altered so that they were completely starved of energy (which is needed to operate the sodium potassium pump). Despite this, the gradient was maintained.
This then begs the question of what could be creating this gradient? Ling made the following observations:
•The gradient was maintained if the cell membrane was removed.
•Producing a membrane with sodium potassium pumps that did not contain a cytoplasm (the inside of a cell) resulted in the gradient rapidly disappearing from inside the membrane.
This suggests that the gradient is a property of the cell cytoplasm rather than the cellular membrane. Interestingly, the identical property has been found in artificial gels, which like the cytoplasm, also generate large amounts of liquid crystalline water:
We show that the gel barrier is able to maintain a stable separation of ionic solutions of different ionic strengths and chemical compositions without any pumping activity. For the Na+ /K+ concentration gradient sustained across the barrier, a negative electric potential develops within the K+ -rich side. The situation is reminiscent of that in the cell. Furthermore, also the advective flow of water molecules across the gel barrier is restricted, despite the gel’s large pores and the osmotic or hydrostatic pressure gradients across it.
This concentration phenomenon has also been observed by other authors:
Evidence for such accumulative condensation was produced almost a century ago when Bungenberg de Jong in 1932 showed that even dilute solutions of polymers, when shaken, coalesced into droplets-then called coascervates-in which the organic matter became highly concentrated. The polymer concentration in such droplets could exceed the concentration in the surrounding bath by as much as 10,000 times. When placed in certain dyes, the droplets became progressively more colored, the intensity often many times exceeding that of the surrounding solution. Thus, gel droplets had the capacity to concentrate certain solutes, as the cell concentrates potassium.
Since gels independently demonstrate many of the inexplicable characteristics of cells, it is thus reasonable to conclude they might share a mechanism for producing the sodium potassium gradient observed throughout biology.
One major challenge for all cells is keeping unwanted things out of their cell’s system. Classically, we view the process of pathogenic substances entering cells as being a product of their interaction with matching receptors on the cells (a world view I believe is emphasized by medical science because drugs can be developed to disrupt this process). I personally believe that this exclusion process by the cell is heavily influenced by charges, zeta potential and liquid crystalline water.
Because liquid crystalline water can for the most part only form on negatively charged surfaces (the same way that water molecules each expel half a hydrogen atom and thereby hold a net negative charge due to the lost hydrogens), almost every surface in the body (and many other natural systems) is coated with negative charge. Since colloidal stability partly depends upon the mutual repulsion created by identical charges, most natural colloidal systems require negative charges to remain dispersed and clump together if excessive positive charges are present (most methods of restoring zeta potential to aid colloidal dispersion operate on this principle).
Pathogenic organisms often impart positive charges to their environment (such as through bacteria decarboxylating the negative charges of amino acids within proteins). This causes proteins and cells inside the body to begin clumping together (the malaria parasite is particularly good at doing this), which both prevents the immune system from gaining access to the microbes and increases their ability to gain access to the cells.
One of the particularly interesting observations made by the original proponents of physiologic zeta potential was that bacterial and viral infections (I believe this was the most studied with influenza) would create a consistent degradation of the physiologic zeta potential. This provides an explanation for why viruses like influenza can be so much more dangerous for the elderly. Someone with a healthy zeta potential can tolerate a minor impairment of it (they will just feel a bit unwell), but for someone who already has an impaired zeta potential (which is a very common consequence of aging), this can be sufficient to pass a critical threshold where severe illness onsets.
One of the earliest distinguishing characteristics of COVID-19 to me was that many patients developed clinical signs suggesting severe zeta potential disruption, something I considered to be highly unusual. A key reason I was concerned about the spike protein long before the vaccines entered the market, was because I had identified it as the likely culprit for the zeta potential disruption. This was due to it being a novel component of the SARS-CoV-2 virus (which differed from that of SARS-CoV-1) that was present on the surface of the virus and which had a high positive charge density. It has since been shown that the spike protein directly induces clumping of red blood cells, likely due to its charge distribution having the potential to create a significant disruption of physiologic zeta potential.
One of the interesting comments I have repeatedly seen stated by both individuals who have taken protocols to improve their body’s production of liquid crystalline water and individuals who have done the same for improving their zeta potential, is that they rarely get sick. This could potentially mean either that the symptoms of infections are much more minor if these physiologic parameters are already in good health (e.g., since you don’t pass a critical zeta potential threshold), or because they directly prevent cells from becoming infected by pathogens. As many factors which positively affect one of the parameters often affect the other (e.g., earthing improves zeta potential and promotes the growth of liquid crystalline water in the body), it’s a bit hard to say which effect ultimately predominates, and a great deal of research for this series was based on trying to answer that question.
For this article, we look at it solely from a barrier perspective, and on that point, there is some interesting corroborating evidence. The earliest one I came across was from the work of Viktor Schauberger (1885-1958), who believed it was critically important for water to travel in a vortexing pattern, something as discussed in part 1, has been shown to increase the presence of liquid crystalline water. One of the many discoveries made by utilizing vortexing systems based on his design for water supplies was that it reduced the growth of bacteria within the water system (e.g., inside pipes).
Other research has directly found that the presence of liquid crystalline water prevents the growth and invasion of bacteria, such as this study:
In a test conducted with E. coli, cells progressively penetrated EZ [liquid crystalline water] over 2 days. Furthermore, EZ-bearing Nafion had 80% less biomass accumulation of E. coli over 2 days compared to an EZ-less, hydrophilic, smooth aluminum oxide surface. This suggests that EZ may represent the first line of defense, spatially and temporally, against bacteria approaching certain hydrophilic surfaces. These findings could have important implications in developing biofouling-resistant material surfaces for applications sensitive to bacterial attachment and biofilm formation.
And this study:
Silk sericin is a globular protein whose resistance against fouling is important for applications in biomaterials and water-purification membranes. Here it is shown how sericin generates a water-exclusion [liquid crystalline] zone that may facilitate antifouling behavior. Negatively charged microspheres were used to mimic the surface charge and hydrophobic domains in bacteria. Immersed in water, sericin formed a 100-µm-sized exclusion zone (for micron-size foulants), along with a proton gradient with a decrease of >2 pH-units. Thus, when in contact with sericin, water molecules near the surface restructure to form a physical exclusionary barrier that might prevent biofouling. The decreased pH turns the aqueous medium unviable for neutrophilic bacteria. Therefore, resistance to biofouling seems explainable, among other factors, on the basis of water-exclusionary phenomena.
Many tissues depend on the presence of liquid crystalline water lining them, and this is likely at least partly, due to the protective barrier they create. Green and Otori in 1970, for example, found exclusion zones approximately 350 μm deep extending from the cornea, and also from contact lens (it’s important to protect the eye from damage, and liquid crystalline water functions as a transparent, well-lubricated barrier, qualities both critical for the eyes). Pollack, approximately 15 years ago, also found identical results next to polyNIPAM, the gel used to fabricate contact lenses.
One of the most important tissues in the body for providing a protective (but well-lubricated layer) is on the endothelial lining of the blood vessels. There, large amounts of blood creating significant shear forces constantly pass over the blood vessels. To this point, Malcom Kendrick has noted that the areas of circulation which experience the greatest shear forces are the most likely to develop chronic damage which eventually leads to heart disease.
The protective coating of the endothelium, the glycocalyx, is structured (due to its high sulfate content) to create large amounts of gel (liquid crystalline water) around it and thus protect the endothelium. Additionally, a thin layer that blood cells cannot enter is known to directly line the walls of even the smallest blood vessels. Similarly, researchers in 2000 observed a 0.4-0.5μm thick gelled layer of water lining the endothelium of healthy capillaries (the smallest blood vessels in the body), and this lining excludes a variety of different substances from reaching the endothelium.
Officially, the vaccine spike protein cannot affect the endothelium because it lacks a functional ACE2 receptor that the COVID-19 vaccine uses to enter it. Although this is the case, there are many other ways that the spike protein can still attach to the endothelium (e.g., the spike protein can still attach to the receptor, it directly binds heparin in the glyocalyx, and if it is produced from inside the endothelium by the vaccine, it has effectively entered the endothelium). Since autopsies of individuals suspected to have died due to vaccination have revealed profound damage to the endothelial layer, this provides strong evidence that the spike protein in one way or another has the ability to penetrate the normally protective barrier there.
Many have suspected that this is partly due to the highly positive charge that the spike protein (and the sometimes positively charged components of the lipid nanoparticles) carries, as these positive charges can penetrate the liquid crystalline water barrier protecting cells like the endothelium. I believe it may also be due to the spike protein’s disruption of zeta potential, as other states associated with increased blood viscosity (e.g., uncontrolled diabetes) are known to damage the endothelium and accelerate its progression to heart disease (along with increasing one’s risk for diabetes).
Note: since the exact effect of the lipid nanoparticles is a complex and unclear subject, it will be discussed in a future article.
Although liquid crystalline water is necessary for life, too much of it can also be problematic. For example, if the entire cell was covered with gel-state water, it would not be possible for many things that needed to enter the cell to enter. To solve this problem, areas of positive charge exist in the membrane (e.g., Pollack argues that this is a key reason for the placement of metal ions within the cell membrane), which prevent liquid crystalline water from forming over the area and thus creating an open passage for water to travel through.
Note: although we are taught to think of the cell as a fluid mosaic of phospholipids with proteins here and there, approximately 50% of the cell membrane is typically composed of proteins (and it can sometimes be even more).
In most cases, however, the body relies upon being able to transform water between its gel (liquid crystalline) state and a sol (bulk water containing colloidal suspensions) state as needed. However, while recognition of the fundamental importance of phase changes in cellular biology has gradually increased over the last 70 years, the majority of the scientific field is still wedded to the fluid mosaic hypothesis, which believes that the cellular membrane always being a liquid is a central dogma of structural cellular biology.
Briefly, gels (both natural and synthetic ones) typically have a temperature (its transition point) where the gel will undergo a rapid transition from one state to the other (explained further here). As far as I know, in all cases, the hotter temperature will correspond to the sol state (H2O water with suspended colloids) and the colder one will correspond to the gel state (H3O2). Additionally, increased pressure will also promote the gel state:
“Biological activity almost universally requires that membranes be in their fluid state; in many cases, just above the transition point”
Gels throughout the body, in turn, are designed to have a phase transition point near the normal temperature of the body. This may explain why most mammals and birds maintain their temperature between 35°– 42°C (95°– 107.6°F; the normal temperature for humans is 37°C or 98.6°F), an otherwise curious fact given how much their bodies and environments differ (e.g., a tiny bird in the desert vs. a blue whale). Additionally, this temperature extends beyond warm- blooded animals. A tuna fish, when active, maintains the temperature of its body at 30°C or 86°F (the ocean is much colder) while many reptiles maintain their body temperature near 40°C or 104°F (by doing things like seeking out the sun). Many insects also cannot fly until they have warmed up to a similar range of temperature (e.g., 38°C or 100.4°F for bumblebees).
Similarly, the phase transition point is heavily influenced by the materials that the gel builds from (e.g., components of the cell membrane). Cold-blooded animals in turn have been observed to change the composition of their cell membranes in response to their environment.
In one experiment, Calotes versicolor (the common garden lizard), was acclimatized to 16°, 26°, and 36°C (60.8°, 78.8°, and 96.8°F) for a period of thirty days. The lizards exhibited dramatic changes in the concentration of major phospholipids (components of the cell membrane) such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, cardiolipin, and sphingomyelin. Temperature acclimatization has also been observed to vary the lipid composition of carp muscle microsomes along with carp blood cells, and seasonal variation has been observed in the lipid composition of brains of rainbow trout.
Once the gel is held near its transition temperature, it is then necessary to change exactly where that transition temperature is so water is in the appropriate phase for the current physiologic needs. This is primarily done within the body by changing the electrolyte composition of the area, as strong cations (positively charged ions) destroy gels, while strong anions (negatively charged ions) reinforce them. As far as I can tell, the effects of these ions correlate to the high valence (charge) anions (-) that most effectively support physiologic zeta potential and those high valence cations (+) which most effectively destroy it.
The primary cation element which the body uses to shift the transition temperature in order to destroy gels is calcium (Ca). To illustrate the effect of calcium graphically (note how a sudden critical threshold of Ca concentration is reached):
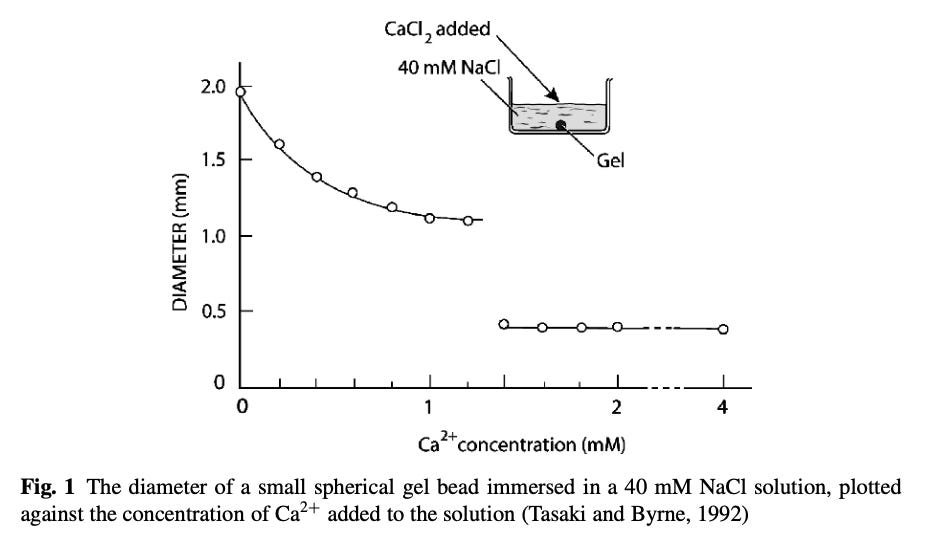
These results also showed how the higher valence cations, Ca2+, cause smaller gels to form relative to low valence cations Na(1)+.

Once you put all of this together, you effectively get a situation like this within the cells which allows small secretions of calcium to shift its water from a fluid to a gel state.
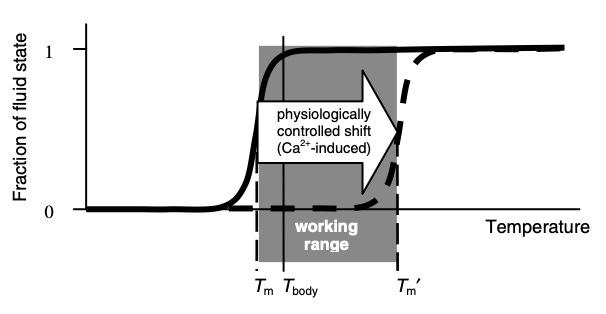
Conversely, if the body can disperse the gels, it also needs a way to recreate them. The primary strong (high valence) anion it uses to build them are the phosphates in adenosine triphosphate (ATP).
When we consider this alternative function of ATP, we should consider some of the ideas first proposed by Ling (see 8.4 on page 178). They suggest that much of the energy obtained from ATP arises not from the energy within its bonds being broken, but rather from it inducing water to form a liquid crystalline structure. In essence, much of the energy generated from ATP ultimately arises from the radiant energy which triggers these polymerizations (gels).
Phase transitions are very common in cellular biology, and while the field does not place a heavy emphasis on their importance, Pollack’s central argument in his first two books is that phase transitions are necessary to explain many functions of the cell.
One of the primary uses for gels within the body is to rapidly expand gels and create effects from that expansion. For example, cells frequently excrete small spheres (known as vesicles) containing important components, which upon exiting the cell rapidly expand. Isolated mucin-producing vessels for example undergo a 600 fold expansion in 40milliseconds.
Prior to exiting the cell, the internal components of the vesicle are cross linked together by Ca2+ (remember, higher valence cations, through crosslinking gel proteins, will limit gel formation while monovalent cations instead tend to expand them). Once secreted from the cell, Ca2+ is removed and the gel rapidly hydrates in the Na(1)+ solution surrounding each cell [note: gels will not rapidly expand in distilled water, and it has been observed that liquid crystalline water’s formation is also diminished within deionized water].
Note: Some vesicles utilize local environmental factors to change their phase. For example, histamine is a monovalent cation (charge of +1) at a neutral pH, but becomes a divalent cation (charge of +2) in an acidic pH. Researchers were able to show that histamine in an acidic environment (due to its high valence state), causes shrinking of mast cell granules, while histamine in a neutral environment allows their expansion.
One of the most common problems for which patients seek out physicians are musculoskeletal pain originating from excessively tense muscles. For this reason, I have looked at a lot of different approaches for addressing those issues. Although I have found a few that I feel are remarkably effective, I commonly find that there is no existing model within muscle physiology that can explain why many of the treatments I know actually work.
One of the major problems with medicine (and often science) is what I term the “mechanistic trap.” Briefly it means that if there is not a mechanism to explain why something works, then it is assumed to be a hoax and thought to not actually work. I think this bias is quite silly since many of the models we utilize to explain why drugs work, don’t actually make sense. There are many cases of a previously accepted model for a drug’s mechanism that was discarded and replaced with another, and I know of many more examples where an unorthodox model appears to provide a much better explanation for the mechanism of a therapy.
Note: This also holds true for many alternative non-drug therapies.
Unfortunately, it is nearly impossible to have the FDA (or many conventionally trained physicians) be open to considering a therapy for research unless a biochemical mechanism for it can be advanced. I believe that this is because pharmaceutical drugs, as they are designed to target certain enzymes in the body, fit into our rigid mechanistic paradigm, while therapies with a broad spectrum of advocacy, which cannot be linked to a specific biochemical receptor, do not.
Given all this, I was overjoyed to discover through Pollack that many aspects of the existing model for muscle physiology don’t make sense, and the expected results of the model do not occur when experiments on muscles are conducted:
However, an alternative model for muscle physiology also exists. Before we go further, I will admit that I am slightly biased here because this model provides a mechanism for some of the therapies I have repeatedly observed treat overactive muscles.
When I teach students and patients about muscles, I explain to them that muscles can only contract and not lengthen. Anytime a part of the body nonetheless “lengthens” (e.g., reach out and touch something out in front of you), this occurs because a muscle contracting on the other side of a joint will cause the joint to lengthen.
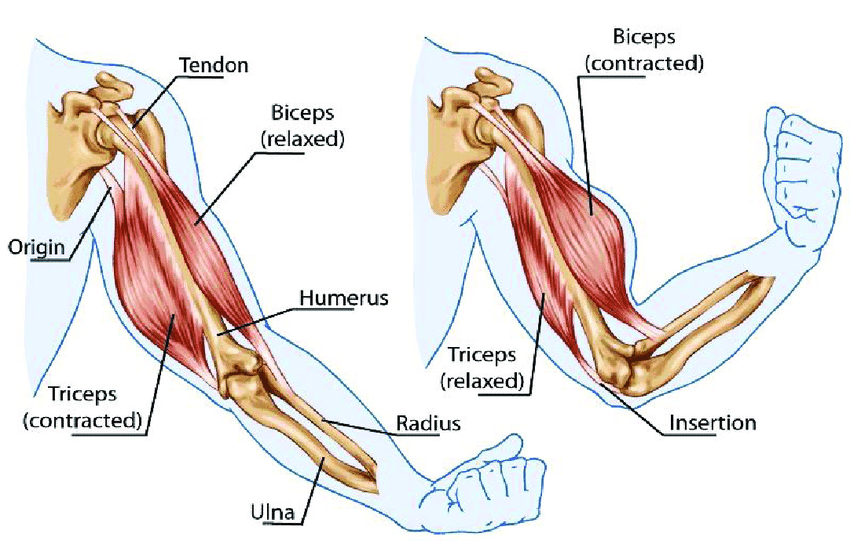
In order for the system to work, it always has to have one muscle that relaxes perfectly in synchrony with the opposing muscle that contracts. Unfortunately, in many cases, the relaxing muscle fails to relax, and instead causes a chronic dysfunction to occur within the muscle unit. From Pollack, however, I learned I had actually made an erroneous assumption for years.
Pollack’s model was based on the observation that relaxed muscles contain a large amount of structured water, whereas contracting muscles primarily contain water not in a semisolid gel state. Since it is known that muscle firing follows the nerve signal directing calcium (Ca2+) ions to enter the muscle, this argues for a distinctly different model to explain muscle physiology.
Conversely, it is also known (from the experiment previously described in the gradient section) that while muscle cells that have been cut open can retain their potassium to sodium gradient while in a salt solution, the parts that lack ATP are unable to. This suggests that ATP is necessary for the liquid crystalline state of water to be present, as it excludes sodium ions from entering the muscle cells. Similarly, it is also known that without ATP, muscles will remain stuck in the contracted form and be unable to lengthen, something that occurs shortly after death (termed “rigor mortis”).
Note: one of the compelling anecdotal stories I have heard over the years is that when severely vaccine-injured children die (e.g., those with lifelong debilitating autism) rigor mortis sets in much faster for them. Those who observed this suggested that these children had a chronic energy deficit, and their bodies were struggling to make enough ATP to get by. I think this is very possible, but it could also be indicative of poor zeta potential. I suspect this might also be seen with other chronic debilitating illnesses, but I have not interacted with the people who would have observed if it did in fact occur.
Pollack also observed that muscle changes in response to environmental factors followed a critical threshold being passed. This seemed to suggest to Pollack that a phase change was occurring within the muscles.

So what is Pollack’s model?
The muscles are designed to form large gels around them, and as liquid crystalline water within them expands, it stretches the uncoiled proteins in the fibers into helixes (until cross links in the muscle prevent further stretching). When the muscle needs to contract, calcium ions enter the muscle, and eliminate the gels, and the proteins no longer being lengthened elastically snap back to their original uncoiled configuration. In order for the gel to rebuild, ATP is required to break the cross links created by calcium to trigger a phase shift that reconstructs the gel.
Thus, the strength of a muscle is a product of the potential energy created by liquid crystalline water’s storage of radiant energy being released. Or put differently, the strength of muscles actually lies in their ability to spontaneously lengthen, something I had previously assumed they could not do. This physical mechanics of this entire process helps to explain many of my observations about muscles:
Large mechanical shear causes the cytoskeleton matrix [another key structure that expands and contracts in response to phase changes] to fluidize, a phenomenon similar to physical rejuvenation in SGM’s [certain synthentic materials]. This fluidization is followed by a slow scale- free recovery of mechanical properties, a phenomenon similar to physical aging in SGMs. Surprisingly in response to a transient stretch, the cytoskeleton fluidizes in a pattern that is universal for different cell types. This finding implicates mechanisms mediated not so much by specific signalling pathways, as it is usually assumed, but rather – as we explain below – by non-specific actions of physical forces.
Many other structures also have the ability for their internal gels to cause expansion and contraction, but unlike skeletal muscles, in most cases, this expansion and contraction is not constrained by the cross links between muscle proteins. Smooth muscles, as well as contractile rings, synthetic actin gels (actin is one of the most common contractile proteins in the body), and other organelles with few covalent cross links and semi-random structures, can thus shorten massively.
Lastly, it has also been observed that changing the surrounding concentrations of salinity around other fibers (e.g., collagen) can cause them to expand and contract to the point they can generate a force yielding contraction like a muscle fiber.
There have been three central questions I have thought about while writing this series.
The first is that quite a few traditional medical systems believe that an inherent expansive force exists throughout the body which plays a key role in providing its vitality, whereas when it is compromised, a variety of diseases set in. I believe that this is the case, as with practice, it becomes possible to recognize where that expansion has been replaced with a tight compacted space, and over the centuries a few really intriguing approaches have been developed that appear to address this problem and restore a patient’s vitality. Unfortunately, there is no existing model to explain where this expansive force comes from.
The second is what exactly is the relationship between zeta potential and the presence of liquid crystalline water in the body (I have spent more time than I can count trying to understand this). As mentioned before, virtually all natural colloidal systems depend upon mutual negative charge for dispersion. Similarly, liquid crystalline water is typically negatively charged and formed on negatively charged surfaces. The reason why calcium (which impairs zeta potential) so effectively condenses the protein matrixes that give rise to gels (thereby destroying the gels), is due to those proteins containing negative charges that calcium can effectively attach to.
The third is where motion arises from within the body, as impairment of any type of circulation (there are many different ones within the body) is one of the most common causes of diseases.
In this article, I have tried to illustrate the structural consequences of liquid crystalline water within the body, and it is my hope that I have answered the first question. In the rest of this series, I will explore those next two questions along with the other ways that structured water can convey information and generate energy within the body (e.g., with the nerves), and then conclude with the approaches I know of for increasing generation of this phase of water within the body.
I hope you are finding this series interesting and that even if you can’t understand everything (I am trying to strike a good balance between making the subject understandable and having the information necessary for more skeptical parties), I hope there are still some useful tidbits you can take home from this. Since this is a bit technical and these take a long time to write, I will be returning to the vaccine subject for a bit so you can digest the first two parts of this series.
Although I did my best (I reread everything here and more for this series) as I am not Pollack, it is very likely I am misinterpreting some of his work. If you are familiar with any of these subjects, I would greatly appreciate knowing if you believe I have made any significant mistakes here.