
What Is The Forgotten Side of Water?
by | Mar 4, 2023
In a recent article, I shared a consistent observation I have made about science: whenever a complex phenomenon exists, science will typically default to comprehending it through a model that artificially simplifies the phenomenon into something that can be easily defined within a more rigid framework. I shared this idea because that article’s focus, the pleomorphic (occurring in various distinct forms) nature of bacteria, is one such example of a complex phenomenon which has been replaced with a much simpler paradigm (bacteria being viewed as having a predominantly monomorphic nature).
One of the areas where this denial of complexity is most commonly encountered is with water, which we learn to treat as a generic liquid even though it is anything but that. As a result, most people are unaware of the innumerable ways water’s behavior fails to match what conventional models predict about it. Despite this widespread lack of knowledge about the true nature of water, throughout the ages, numerous scientists have broken from their peers and produced a variety of remarkable discoveries about it.
One such example is German naturalist Viktor Schauberger (1885-1958), who recognized that water, rather than traveling linearly, moves in spiraling currents and vortices that facilitate many of its properties. He designed a variety of innovative devices that worked in harmony with these properties of water (see this video). Long after Schauberger, a team of Russian physiologists led by А. I. Goncharenko, through a large number of flash-frozen cross sections of tissues, likewise discovered that blood travels in spiraling vortices, and that this behavior helps to explain numerous aspects of the circulatory system.
Note: The Russian discoveries, which will be discussed in a future article, also match traditional Chinese views of the heart and circulation (along with those held by a few other schools of holistic medicine)..
Is Water in the Body Homogeneous?
Within the conventional biomedical model, water is thought of as a uniform, evenly mixed (homogeneous) substance that exists as an aqueous solution that facilitates the random mixing of chemical reactants necessary to produce the biochemistry of life. Homogeneity is assumed to occur within any contained water compartment (e.g. inside a cell, inside the blood stream, inside the gallbladder), because with this assumption, water takes a passive role and thus, greatly simplifies the process of modeling complex biological processes.
However, when you dig into this, you often find water and fluid systems (e.g., blood) are anything but homogeneous. The previously mentioned Russian researchers, for example, made an excellent case that blood traveling through blood vessels in a vortexing motion concentrates its components into the center of its vortex (thereby reducing their drag on the periphery of the blood vessels). and that different compositions of blood concentrate in different parts of the body.
Similarly, to quote one book covering the subject of another mostly forgotten medical therapy:
Even venipuncture triggers physiological changes affecting the whole organism and lasting hours or days. These responses include transmission of electrical stimuli throughout the fascia and changes in venous leukocyte counts, immunoglobulins (A, M, and G), blood cholesterol, oxygen saturation, electrolyte levels and pH values.
Even more interesting is the discovery that these measurements (in venous blood) show body asymmetry corresponding to the site of puncture. This phenomenon correlates with similar findings in venous blood in the presence of unilateral inflammatory processes…Some of the studies cited above were performed in a search for a reliable method of detecting interference fields. These studies revealed that venous chemistry is altered on the side of the body that harbors an interference field. In fact, not only is it altered, but the way in which it is altered depends on the person’s general health and the presence of various disease states.
Disclaimer: I am unsure if follow-up research was ever performed confirming the above findings. Similarly, with the Russian research, I have read their results and have mutual friends who know the researchers, so I am inclined to believe their work is valid, but I am unaware of any English peer-reviewed publications where the data I’ve received was published.
Colloidal Stability
When a substance is mixed into water, it can fail to mix (e.g., oil floating to the top or sand sinking to the bottom), or it can dissolve (e.g., salt in water). Sometimes when the substance cannot mix (it is insoluble), rather than gravitationally separating by density, it will instead form a colloidal suspension.
Anytime a substance is mixed into water, there will always be forces that attract its particles together (e.g., the Van der Waals force and gravitational separation) and forces that repel them. When the repelling forces are sufficient to overcome the attractive forces, the particles will become suspended and a colloid will form. The behavior of chia seeds (an insoluble substance) in water help to illustrate this concept:

In the above image, if you look at the individual seeds present, you will see that a (hydrophilic) gel has formed around each seed, and this creates a barrier that prevents the seeds from coming together and separating by gravity from water. However, in many cases, this surrounding gel does not sufficiently form, and gravity separates the seeds from the water (the example shown below mirrors what can happen with red blood cells in the body).

Note: Although chia seeds provide an excellent way to illustrate this concept, the above pictures are typically not classified as colloidal systems (colloidal particles are much smaller and thus not possible to see without magnification). However, the gel surrounding a chia seed soaked in water is considered to be a colloid.
The typical repellant forces which keep colloidal particles from clumping together are primarily electrical charges (similar charges repel each other), molecules providing steric hindrance (which are beyond the scope of this article), and hydrogels (like those seen around chia seeds).

Most biological systems are colloidal suspensions (e.g., proteins are colloids suspended in water; blood is a variety of colloids like red blood cells suspended in plasma) that depend on mutual negative charges for dispersion. Biology depends upon a precise balance between the attractive and dispersive forces within these colloids, and frequently humans run into issues arising from external factors causing excessive colloidal attraction (agglomeration), which I believe to is what occurs in the initial stages of blood clotting.
For example, one of the best models I have seen for explaining many of the consequences of vaccination is that they reduce the electrical repulsion present between red blood cells, causing them to clump together and create microstrokes in areas where those blood cell clumps are now too large to travel through the blood vessels. The COVID-19 spike protein has a positive charge that appears to inhibit the electrical dispersion naturally present within the body, and we have found treatments aimed at improving a patient’s colloidal dispersion (which depends upon negative charges) mitigate symptoms of vaccine injury.
Blood clumping from poor colloidal dispersion is one of the most common mechanisms seen behind a variety of diseases, and I previously discussed some of it here.
Gel-State or Interfacial Water
The gelatinous coating surrounding chia seeds, for example, also provides an excellent illustration of how water can reach an intermediate state between being a solid and liquid where it behaves more like a gel. Hydrogels (water gels which can sometimes be composed of 99.9% water) exist throughout nature, and this viscous form of water is vital to biology and the normal function of the human body.
Water also can become more “solid-like” in other situations. Consider the experience of touching the surface of water—there the water is somewhat solid, but once you break the surface tension and enter bulk water, much of that solidity disappears.
Many organisms depend on surface tension (e.g., water striders and certain lizards use it to “walk” on the surface of water). Conversely, in certain fields like competitive diving, at greater heights it is sometimes necessary to reduce surface tension (so there is less force experienced when impacting water) by bubbling air throughout the pool. Similarly, many have theorized this is what cause ships to be lost in the Bermuda Triangle, as methane outgassing from the seafloor effectively breaks the surface tension of the water above and causes ships to lose their ability to float.
A key area where water commonly develops a partial solidity is at its interfaces with particles or surfaces contacting water, especially those containing charges. Water in this region is often referred to as interfacial water. The technicalities of interfacial water are a bit too complex for the scope of this article, but the important point is that they essentially build on the previously mentioned points. For example:
The perturbations of dynamical properties of interfacial water are consistent with structural perturbations and strongly depend on the substrate; water shows faster tangential diffusion near surfaces that do not attract water and slower tangential diffusion near attractive surfaces.
Note: for those interested, much more about interfacial water is explained in this article and in this article.
Liquid Crystalline Water and Cells:
Classically, we believe that cells are liquid bags whose contents are dictated by proteins on the membrane of the cells (e.g., the sodium-potassium pump that concentrates potassium inside the cell). However, since microscopes were first developed, an alternative worldview also was developed that indicates that the water inside cells is very different and alternates between behaving in a gel-like fashion and behaving like a normal liquid.
Since this concept is not formally recognized by science, a variety of different terms have been independently coined to describe this state of water including: Confined Water, Exclusion Zone (EZ) Water, Fourth Phase Water, Gel-State Water, Interfacial Water, Liquid Crystalline Water, Semi-Solid Water, Structured Water, Surface Associated Water, Water, and Vicinal Water. I personally think “liquid crystalline water” is the most accurate, while the bolded ones represent the most commonly used terminology.
- This concept is important as it helps to explain many puzzles of cellular biology, such as:
- Where does cellular integrity come from? If cells are just bags of fluid, it should be easy to “pop” them and have them rapidly drain out, but in reality this rarely happens.
- How do cells maintain their internal composition despite the large amount of energy necessary to maintain that gradient?
- Do other energy sources exist for cells?
- How can cells circulate their internal contents so that the biomolecules arrive at their appropriate location?
- Are enzymes sufficient to create the speed of molecular reactions necessary to sustain life within a cell?
- How do cells rapidly communicate with each other from a distance?
- What drives microcirculation?
Note: The conventional models explaining the functions of muscles and neurons also have a significant number of inconsistencies that are addressed by this alternative water model.
The best summary I have found of the history of these observations and their implications is found within this book from 2003. For those interested in the forgotten history of these alternative models (which I think is extremely interesting), I have included the entire section. For everyone else, please skip ahead to the next section as what follows is quite technical; I included it because most of this is quite difficult to find online.
What follows was originally written by Marco Bischof (note: for this part and the next, I removed most of the names and references to shorten it—many scientists have spent their careers studying this subject):
Since the 1950’s a number of alternative conceptions have developed, which trace their origin to early observations of the gelatinous (colloid) properties of living matter, and of the anomalous behavior of water associated with certain biomolecules. Like other biologists before him, Dujardin in 1835 recognized that entity as a “living jelly” that since Purkinye (1839) [has been] known as “protoplasm“. After the introduction of the concept of the “colloid” by Graham in 1861, many biologists and physiologists have conceived of the protoplasm as a hydrated polyphasic colloidal system. In 1908, and in the 1920’s, researchers suggested that the potassium ions in cells, the most abundant intracellular ions, existed in a bound and unionized form.
Schade in 1908 recognized the cell as a polyphasic colloid and wrote in his pioneering book on “The Physical Chemistry in Internal Medicine” that “The body of the cell is neither uniformly solid nor uniformly fluid; each cell rather forms a (…) ‘microheterogenous system’, a mixture of gel-like and sol-like masses and true solutes in a common medium, the colloid nature giving the characteristic feature to the whole as well as to its parts.”
Fischer postulated in 1910 that living cells, together with their intercellular substance, form a water-binding colloid that cannot be compared to a suspension or solution of molecules in water, but rather is a “water-free” hydrated system in which the water is not free, but bound to its colloids, mainly proteins in the form of alkaline and acidic proteinates with a high hydratation potential. In 1938, Fischer and Suer suggested that protoplasm was the “union of protein, salt and water in a giant molecule.”
In the view of Schade, Fischer and many other biologists of the time, the water in the cell and in the extracellular spaces was not free but existed in the form of “bound water”, also called “Schwellungswasser” (swelling or imbibition water). The phenomenon of the strong water affinity of certain biomolecules was first discovered in 1848 Ludwig and again observed in 1917 by Katz. [Then, in the 1930’s and 1940s these perspectives were pushed out by the conventional model].
In 1951-1952, Troshin presented his studies on cell permeability which provided the foundation for his “sorption theory” of the cell. Like the other variants of the “phase theories” or “coacervate theories” proposed as alternatives to the membrane theory, this was based on the interaction of macromolecules and ions with cell water and the protein-lipid membrane. Troshin pointed out that in contrast to the low-protein extracellular medium, the interaction of electrolytes with proteins in the cytoplasm is so strong that ionic mobility and therefore electrogenic activity of ions is strongly reduced; the cytoplasm forms a “complex coacervate” in which proteins, ions and most of the water are strongly bound.
Cytoplasm and extracellular medium therefore form two distinct phases.
A coacervate (a concept introduced by Jong in 1929) is formed when hydrophilic proteins, together with water, form droplets and amoeboid masses that are distinctly demarcated from the ambient fluid. It encloses 80-90% water and remains hydrophilic. Coacervation depends on a strong enough dipole moment and electric charge of the proteins and on the electrolyte content of the medium.
Through coacervation the protoplasma obtains the property of liquid-crystallinity (recently the topic of liquid-crystallinity, first discovered in 1888 as a property of living systems has been again discussed by several authors – see Mishra, 1975, Ho, 1996). Troshin’s work was then followed up in Germany.
The most momentous of these alternative cell theories was the “Association-Induction Theory” put forward at approximately the same time by Ling. It was then extensively tested in the 1960’s and 1970’s with newly developed Nuclear Magnetic Resonance (NMR). The subsequent invention of Magnetic Resonance Imaging (MRI) by Damadian actually was a result of Damadian’s testing of the Ling theory of cell water, when he discovered a difference in NMR signals from water in cancer cells to those from normal cells.
The insight that any theory attempting to explain the unequal distribution of ions between the inside and outside of cells, the dynamic aspects of solute and water exchange and the bioelectric phenomena of cells requires knowledge of the physical state of water in the cells, has stimulated research on the role of cell water and and its supramolecular structure in the following decades, especially after these concepts first reached a wider scientific audience and recognition in 1965 at a conference on the biological role of water structure in the New York Academy of Sciences.
The phase theory of the cell assumes the existence of aqueous compartments (phases) in the cell with distinct metabolic activities, and the exclusion of solutes (e.g., sodium), to some extent, from the cell by the changed solvent properties, and also predicts that changes in the physiological state of the organism can alter the physical properties of cellular water.
The concept of a highly structured cell is also strongly supported by the recent discoveries of the subtleties of cellular architecture…Today, it is clear that we have to view the cytoskeleton as a highly structured three-dimensional network of these elements which is very dynamic and labile. Its elaborate structure is very easily disassembled under the influence of small perturbations, and thus oscillates between a gel state in which the structure is up and visible and a sol state in which it is dissolved.
The discovery of the importance of water structure for the understanding of cell and organism is also significant in connection with the evidence we will describe later, for a role of the structure of cellular water in the reception of environmental information, including ambient electromagnetic fields, by the humoral system formed of body water and extracellular matrix.
According to Kraus, the main work of the organism consists of swelling and de-swelling; the transition from the wet to the less wet state performing the work. Kraus saw the stream of water through the body, driven by the dynamics of protoplasm, as one of the major features of the living state, and he considered the degree and the distribution of water saturation and the turgor of the tissues as a measure of life. One of the important elements of the “vegetative system” was thus the “vegetative streaming” of the body fluids in the vascular system, the lymph system, the interstitial spaces (connective tissue) and the cell. However, “the colloidal biosystem is not only driven and regulated by water, but also by specific salt effects respectively by electrical interface potentials”, and thus the antagonism of electrolyte cations and anions was important.
The colloid scientist Thiele, demonstrated in work spanning the time from the early 1940’s to the late 1960’s that the interaction of complex colloidal gels with ions is the basic structuring mechanism in organic structures, especially in connective tissue.
What follows was also sourced from the previous 2003 book and was originally written by Roeland van Wijk (it was also shortened for this article).
Sherrington in 1938 stated “Although it is fluid and watery, most of the cell is not a true solution. A drop of true solution, of homogenous liquid, could not “live”. It is remote from “organization”. In the cell there are heterogeneous solutions. The great molecules of proteins and aggregated particles are suspended not dissolved. A surface is a field for chemical and physical action. The interior of a pure solution has no surface. But the aggregate of surface in these foamy colloids which are in the cell mounts up to something large. The “internal surface” of the cell is enormous. The cell gives chemical results which in the laboratory are to be obtained only by temperatures and pressures far in excess of those of the living body. Part of the secret of life is the immense internal surface of the cell.”
The drop of polyphasic colloid is a mixture of different environs; some areas are rich in particles, others are not. Water serves as the dispersion medium or continuous phase and protein, nucleic acids, carbohydrates, lipids and ions are the dispersed particles. Since then, many researchers have speculated on the association of enzymes with the vicinal water and with other proteins, including the filaments, have suggested that the water vicinal to the protein molecules was structured differently than normal water. The macromolecular network of the protoplasm and the enclosed water interact in a mutual manner. Thus, bio-polymers have an effect on the structure of water, while the water structure is significant for the protoplasmic organization.
Bio-polymers have an indirect effect on water. Bio-polymers characteristically present to water both charged and hydrophobic surfaces. Globular proteins in solution, for example, bury much of their hydrophobic surface area internally. Nearly all charged and hydrophilic groups, which endow the large molecules with solubility in water, are on the external surface. Inevitably, therefore, those patchy surfaces generate patchy interfacial water structures: zones of dense water grow round the charged groups, while zones of low- density water grow round the hydrophobic regions. These zones of water have consequences for the solubility of small molecules as well as for the macromolecular organization.
The significance of water structure for the protoplasmic organization is then also evident. If water exists in different hydrogen-bonded states in different regions of the protoplasm, both protein conformations and aggregation/deaggregation states are likely to be affected. Low-density water [note: I believe the described water is “high-density” not “low-density”] selectively accumulates chaotropic ions (like Cl-, K+, charged amino acids, and glucose). It selectively excludes highly hydrated ions (Mg2+, Ca2+, H+, Na+), and predominantly hydrophobic molecules. It follows that the buried hydrophobic proteins can more readily emerge, and the proteins unfold, when the surrounding water is weakly bonded. Conversely, stabilization of the native folded conformation and aggregation of monomer proteins by hydrophobic interactions are promoted by strongly bonded water.
In a cell with only ions as osmolytes, extreme forms of water that are deleterious to enzyme stability and function coexist. Water adjacent to the filament is probably so weakly bonded that internal hydrophobic moieties emerge and enzymes unfold and denature. However, in case the macromolecular network of the protoplasm contains not only ions, but also compatible solutes as osmolytes, the excess osmolality is offset, to a degree, by accumulation of compatible solutes into the strongly bonded regions adjacent to hydrophobic patches of surface and in between filaments. Water near the charges on the filaments can equilibrate with a less drastic increase in density and reactivity, while the rest of the water maintains a rather open structure, because of the hydrophobic portions of its dissolved solutes. In this case, enzymes still accumulate into the more reactive water associated with the filaments, but they do not denature.
The view of a cell as a highly integrated system of multiple components interacting with one another leads to the question on the quantity of water in the vicinity of surfaces. It is evident that moving away from the surfaces and charged groups, the cell water begins to act more like free water. This is a continuum theory; it does not imply that there are two kinds of water but rather that water changes. The early evidence already indicated that the great majority of intracellular macromolecules, including enzymes are probably not free in solution, but are instead loosely associated with formed structure.
From the known physical properties of extruded protoplasm, which maintains its physical integrity, it is likely to suggest that cell water occupies a large portion of the cell volume in the vicinity of surfaces, where it may have a longer residence time and moves more slowly.
In this respect it is significant that several studies have indicated that water within 25-30 Angstrom of biological surfaces is known to exhibit physical properties that differ significantly from those of pure water. This influence can extend even over 500 Angstrom from such surfaces.
Cells therefore are perceived to need some new strategy for an intracellular directional flow of nutrients. Interestingly, a fluid movement in relative large cells as unicellular Acetabularia cells has received broad attention with respect to the question how metabolic events within these giant cells might operate in a controlled manner. In earlier studies it was generally accepted that there is an upper limit to the size a cell can attain before diffusion becomes too slow a process at the molecular level to satisfy metabolic demand. Big cells, therefore were perceived to need some strategy for dispersal of nutrients, and came up with the process of protoplasm streaming.
However, streaming is seen in large cells for no other reason than that these cells lend themselves to such observation. In fact, similar fluid movement necessarily occurs in all cells. It is evident that if movement occurs in a concerted manner, and all the particles move in a certain direction, these particles can move from one location to another much more quickly than could ever be achieved by diffusion random movements of molecules in a non-stirred environment. Wheatley has pointed up the fact that directionality of the flow of molecules provides information to maintain vital activity. The intracellular directional flow increases the complexity of the organization of the cell.
With such view we are beginning to get a more complete image of the structural and functional organization of the protoplasm. A significant parameter therefore in relation to metabolic activity is the flow. The speed of flow, its control, and its directionality have been long overlooked in favor of concentration at the cell level. At basal metabolic rate, a low flow rate might suffice to keep life going, but for cells to work under extreme conditions it demands that fueling is up-regulated almost immediately. This brings in another factor, dealing with the relationship between structure and function of the protoplasm. If the protoplasmic mass anticipates work, some restructuring has taken place before it actually begins its exertion.
And as soon as it comes into operation the flow automatically is faster in the protoplasm structure. This element of purpose is most appropriately introduced into classical cell physiology by assuming that the cell is directed and effective in particular circumstances through innate reflexes. According to the model proposed in the present chapter, the proper cooperation between cellular processes can be understood if it is assumed that the concerted movement of particles is an essential part of the coherent structure. The directed movement can be seen as reflecting the dynamic coherent domain
Note: The history of liquid crystalline water is also further detailed within this book and this book, both by Gerald Pollack, and this article by Stephanie Seneff.
Exclusion Zone Water
Gerald Pollack became the most recent scientist to take up the banner of studying the unusual properties of water in his laboratory in his search for a unifying model to explain many of the holes within cellular biology. In his 2009 research, he eventually discovered that polystyrene microspheres placed in water did not evenly distribute themselves; instead he found they would be excluded from certain areas (this discovery has been confirmed by many others as well—especially near the surfaces of gels). These areas of exclusion became termed an “exclusion zone” (EZ) and the water within them “exclusion zone water.”
To visually represent what Pollack observed (the black dots are the microspheres):
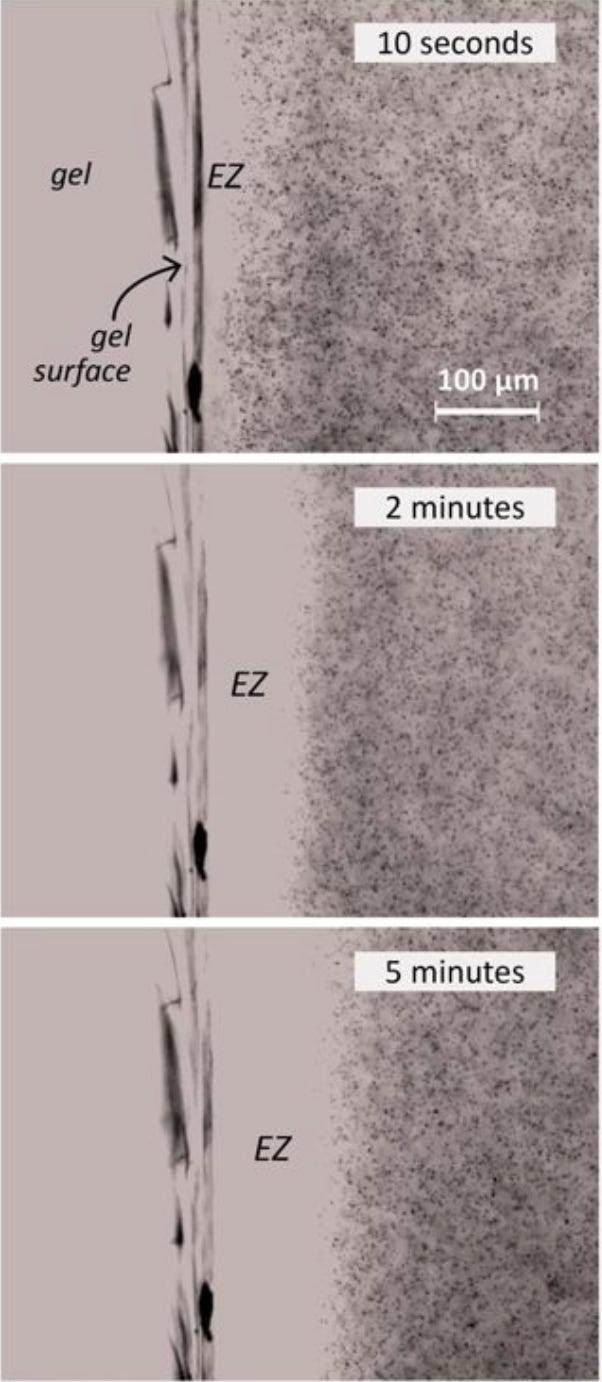
This and a variety of other observations led Pollack to conclude that a structured layer of water was forming at the interface of certain substances placed in water. It should be noted the existing conventional scientific viewpoints support the notion that a few water molecules will cluster together in these interfacial zones, but, nothing on 0.1 millimeter scale that Pollack observed (which requires approximately one hundred thousand times as many water molecules to line up).
This is a brief presentation Pollack gave which touches upon many aspects of EZ water:
In the rest of the article, we will touch upon some of the properties of EZ water, many of which mirror those observed in raw egg whites, one of the most common types of gels we interact with in our everyday life.
Properties of Exclusion Zone Water
In order for exclusion zone water to form, it requires a hydrophilic surface to form upon (this can include individual particles in water), which in most cases must be negatively charged (positive surfaces can sometimes create EZ water, but these EZ zones are weak and easily broken). Once this condition is met, and electromagnetic energy is present (particularly infrared light, which exists everywhere), water will store that ambient energy by assembling itself into layers of offset hexagonal sheets with the formula H3O2.
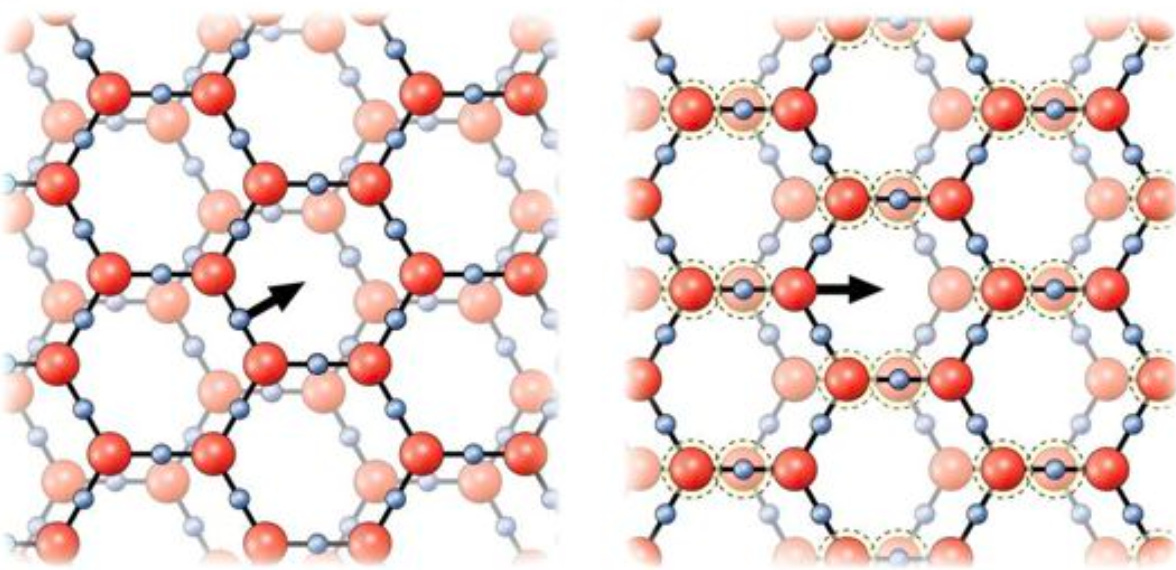
As Pollack describes:
This model yields a stable structure that sticks together naturally. This model yields predictable mechanical behavior: semisolid when left alone, yet able to flow in response to an imposed shear force. Its behavior should resemble gelatinous egg white.
Due to the shape of this lattice, each plane can easily slide past the adjacent layer and slot into the next alignment between those planes (which helps to explain some of the mechanical properties of EZ water interfaces), and electrons can easily travel through the lattice (resulting in it being approximately 100,000 times as conductive as the surrounding unstructured water).
Since this structure is “missing” protons (as it is H1.5O rather than H2O), those protons have to go somewhere—immediately outside the exclusion zone created by this crystalline lattice. For this reason, a region of negative charge can be observed within the EZ, while a region of positive charge (which is also acidic due to it being comprised of protons), can be observed outside it.
Pollack and numerous other researchers have documented the existence of this charge separation, which persists long after the EZ water initially forms (and which has successfully been harnessed to power small electronics). Additionally, they have also demonstrated that a pH change existing concurrently with EZ water, further supports the notion that the lattice is expelling protons that create a charge separation alongside it.
Since the negatively charged region (the H3O2 water) exists in a crystalline structure, it will prevent most substances and ions from existing within it (which, amongst other things, has been utilized to provide an economical means of water purification). Similarly, because this region and its vicinity is more constrained, an increased viscosity exists that slows diffusion of substances within it, and this region can be directly observed by resonance imaging technologies that detect molecular restrictions (NMR and MRI).
In addition to the crystal lattice excluding objects (thus making it detectable on microscopy if microspheres are placed in water), and its periphery being acidic (thus making it detectable with pH dyes), this region also has a variety of optical properties that can be used to detect its presence. EZ water absorbs ultraviolet light (at the 270 nm wavelength), it radiates less infrared radiation than the surrounding water (indicating less movement is occurring within it), and it has a refractive index of about 10% greater than bulk water (which again implies it has a higher density).
By each metric, EZ (liquid crystalline) water has been found to have a higher density than normal water, and it appears to compose the greatest percentage of water at 4 degrees Celsius (this was the same temperature Viktor Schauberger identified as providing the greatest density to water—likewise vortexing water, the water vitalizing method used by Schauberger and Rudolph Steiner also showed to contain a 270 nm peak).
Phase Changes and Liquid Crystalline Water
Some of the most consequential aspects of liquid crystalline water are its involvement in the phase transitions of water (e.g., solid to liquid, and liquid to gas), as well as how often biology depends upon bulk unstructured water transforming to liquid crystalline water (and vice versa). Because of the influence of the classical phase changes of water, liquid crystalline water will form in conjunction with the phase change, and factors that modify the presence of liquid crystalline water will also change the temperature at which water will freeze or boil.
For example, many organisms can survive living in ice cold environments by manipulating the gels (EZ water) inside their bodies so that it (and the organism) does not freeze. Similarly, the properties of EZ water can also be taken advantage of to induce phase changes:
Note: liquid crystalline water can is most observable in this video during trick 3.
In addition to the liquid to solid transition (note: there are presently 19 known forms of solid water), Pollack has also provided a strong case that water depends on its liquid crystalline phase to transition from a liquid to a gas, and that many weather patterns (e.g., the existence of clouds) are a consequence of the presence of liquid crystalline water.
In addition to proposing that liquid crystalline water creating the electrical attraction that links water droplets in clouds together, Pollack has also made a good case that other large environmental water systems are also linked together by this form of water (e.g., tsunami waves). One of the more interesting theories he has provided evidence for is that a mosaic lattice of liquid crystalline water will form in bodies of water (in effect, creating a pseudo-solidity there akin to a net being interspersed amongst the bulk water).

I am immensely grateful to Pollack for elucidating the model of liquid crystalline water, and I think about it on a daily basis as I observe my surroundings (especially large bodies of water). However, while its myriad effects in our environment are fascinating, what occurs within living organisms is, in my opinion, by far the most consequential.
For those wishing to learn more about this topic before the rest of this series is released, I would strongly recommend reading Pollack’s two books. If you do so, I would advise starting with his more recent 2014 publication, The Fourth Phase of Water and only after you finish that, using the context it provides to read his previous 2001 work, Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function.
Conclusion
One point a few of my favorite authors (e.g., Pollack on water or Kendrick on the cholesterol hypothesis) have made, is that if you have an incorrect model for explaining a natural process, countless discrepancies will emerge between what your model predicts and what is actually observed. When this happens, the scientific response should be to question the underlying model, but instead, the response is almost always to double down on the existing model and invent elaborate explanations (the “paradoxes”) for each contradictory piece of data that emerges.
All of this is a consequence of the institution of science being transformed from the bold pursuit of uncertainty to an organized institution which seeks to sustain itself and perpetuate the existing paradigms its vested interests depend upon. In essence, science in many ways has become the antithesis of what it was originally, and has ossified into an over-funded institution that no longer prioritizes its original mission of pushing the frontiers of human knowledge.
This phenomenon is by no means unique to the institution of science. I have also seen this institutional bias play out in countless institutions throughout my lifetime (especially when I’ve belonged to it and been on a committee). Many of these institutions were started by brave pioneers who were ultimately successful in breaking from the mold (e.g., one of my friends started numerous successful start-ups and told me he would quit them whenever they had over 50 people because they stopped being fun to work in).
In the last few months, I have observed this process play out both within organizations I am directly involved in (e.g., commercial endeavors or non-profits), and externally with various ones many of you know of which are opposing the COVID-19 mandates. To this point, Robert Malone recently posted an excellent article on the recent events at Project Veritas which mirrors what I have direct knowledge of transpiring behind the scenes in a few other well-known organizations.
In a recent article on the anthrax vaccine debacle, I reviewed many of the statements from servicemen directly affected by those mandates. One, from a Captain seeking to explain why the military sought to cover up these catastrophic injuries and keep pushing forward with the completely unnecessary anthrax vaccines is particularly pertinent to this subject:
Before COVID, the toxic anthrax vaccines were mandated upon the military and disabled over 100,000.
Many tried to fight the mandates and spoke out against the injuries they witnessed in the military.
This statement by a Captain about institutions has always stuck with me. pic.twitter.com/2PcClZCCBY
— A Midwestern Doctor (@MidwesternDoc) March 4, 2023
Many of us believe that the profound issues we witnessed within the institutions of medicine and science throughout COVID-19 did not arise out of nowhere. Instead, we argued they were a consequence of the pre-existing progressive ossification of these institutions as more and more money has flowed into them, and the institutions have rearranged themselves around supporting the institution rather than advancing the mission that each institution was originally founded upon.
I would like to quote some of what Pollack had to say about the state of science ten years ago as he sought to provide an answer for why the science of water has been neglected for over a century, and then ask you to consider how much of this applies to what we have seen over the past three years:
Until the modern era, scientists focused on seeking foundational mechanisms. They tried to understand how the world works. If their efforts uncovered paradigms that could explain diverse phenomena in simpler ways, then they knew they were onto something meaningful. Thus, Mendeleev’s periodic table could predictably account for the multitude of known chemical reactions, and Galileo’s sun-centered solar system obviated the need to invoke complex epicycles to describe planetary orbits.
The pursuit of simplicity seems to have largely evaporated from the scientific scene. In four decades of doing science, I have seen this noble culture yield to one less audacious and more pragmatic. The chutzpah has vanished. Scientists content themselves with short-term gains in narrowly focused areas rather than seeking fundamental truths that may explain broad areas of nature. A quest for detail seems to have supplanted the quest for simple unifying truths.
This minutae-oriented approach seems to me to bespeak a culture gone awry. You can judge this for yourself by considering the results — the scant number of conceptual revolutions that have emerged in the past three decades. I don’t mean technical advances, like computers or the Internet, and I don’t mean hype or promised revolutions, like cancer cures or endless free energy. I mean realized conceptual revolutions that have already succeeded in changing the world. How many can you identify?
Once bold, the scientific culture has become increasingly timid. It seeks incremental advances. Rarely does it question the foundational concepts on which those incremental advances are based, especially those foundational concepts that show signs of having outlived their usefulness. The culture has become obedient. It bows to the regality of prevailing dogma. In so doing, it has produced mounds of data but precious little that fundamentally advances our understanding.
I have tried to reverse this trend in these chapters by returning to the traditional way of doing science. By observing common, everyday phenomena and applying some simple logic, I have sought to answer the “how” and “why” questions that can lead to fundamental truths, while avoiding the “how much” and “what kind” questions that characterize the incremental approaches. I know it is not the fashion, but I think it offers a better path for achieving scientific progress.
He then listed the reasons to explain why the scientific field has been so reluctant to pursue the science of water, a few of which I will quote.
Water occupies a place central to so many natural processes that few people can conceive that the basics could remain open to question. Surely someone must have worked out those basics, probably a century or two ago. This perception keeps scientists away. If anything, their reluctance has only intensified: today’s science rewards those who focus narrowly on trendy areas, leaving little room for questioning widely taught foundational science. Especially for something as deeply rooted and common as water, the incentive to question fundamentals has all but vanished.
A third reason for the slow emergence of such fundamental principles plagues all of science: intellectual timidity. Relying on received wisdom feels safer than dealing with the uncertainties of revolutionary disruption. You’d think that scientists would embrace dramatic advances in fundamental science, but most of them feel more comfortable restricting themselves to minor deviations from the status quo. Scientists can resist revolution in the same way as any other defender of orthodoxy.
A fourth reason is outright fear. Challenging received wisdom means stepping on the toes of scientists who have built careers on that wisdom. Unpleasant responses can be anticipated.
The key to making progress in all of these arenas must include a fresh willingness to admit that the emperor has no clothes. Even the greatest of scientific heroes might have erred. Those scientists were human: they ate the same kinds of food we eat, enjoyed the same passions we enjoy, and suffered the same frailties to which we are prone. Their ideas are not necessarily infallible. It might seem irreverent, but if we hope to penetrate toward ground truth, we need the courage to question any and all foundational assumptions, especially those that seem vulnerable. Otherwise, we risk condemning ourselves to perpetual ignorance.
Pollack has also mentioned that he believes the current scientific grant system (which all non-industry scientists depend upon for their livelihoods) has effectively destroyed the progression of science. This is because it is almost impossible to get approval for a grant exploring any type of revolutionary research which challenges an existing scientific dogma.
When these unorthodox grants are submitted, the experts invested in the previous dogma will be assigned to assess if there is a credible basis for the research (and thus a justification for funding it), and not surprisingly the established will almost always veto any controversial proposal. This is analogous to the current situation with peer-review where scientific articles will only be published if experts invested in the existing paradigm allow them to be published.
This publication bias created a huge number of problems throughout COVID-19, as it became almost impossible for any life-saving information that threatened the narrative to be published. Fortunately, this has inspired numerous teams to try and launch scientific journals that do not cater to the orthodoxy (something Pollack actually did 15 years ago).
Note: It should also be mentioned that The Real Anthony Fauci makes an excellent argument that Fauci, through his position at the National Institutes of Health, has effectively hijacked the entire grant system and used it to blackmail our nation’s public scientific system into only producing research which supports industry narratives.
Lastly, I would also go even further than COVID-19 and argue that these principles help to explain why so many critical facets of medicine have become forgotten. In the past (e.g., during the 1918 influenza), physicians working alone would frequently make remarkable discoveries they shared with their colleagues in leading medical journals of the era (many of which I read to help develop my initial treatment protocols for COVID-19 at the start of the pandemic).
Now, the only place where that foundational research can proceed is within the disappearing laboratory of clinical practice (which is constantly besieged by regulatory pressure which makes independent practice outside of the corporate medical system extremely challenging). Likewise, once conducted, it is almost impossible for this form of research to be published in the medical literature now and I only have access to the remarkable discoveries it unearths through personal connections to the researching clinicians (note: this conundrum is discussed further in this article).
Now that the initial concept has been established, in the next part of the series, we will explore how biology and fluid movement fundamentally depends upon liquid crystalline water. Following that, in the final part of the series, I will discuss known methods for increasing liquid crystalline water within the body, how the spike protein affects this phase of water, and the relationships between zeta potential colloidal stability and liquid crystalline water.
Lastly, since unorthodox ideas about water have been always raised strong objections from the scientific community, numerous attempts have been made to debunk the concepts outlined in this article. In the spirit of sharing both sides of the debate, while I do not completely agree with it, I will also share the most comprehensive attempt I have come across challenging the ideas laid forth here.
I apologize for the delay in publishing these (I try to write 1-2 articles a week). I realized after I started this series and sought to clarify some gaps in my knowledge on the subject that I had to research far more than I had originally anticipated (I would estimate I ended up reading approximately 1000 pages) for this series, something I was happy to do as I believe this is a critical topic to present correctly.




0 Comments